The right tools drive stem cell research forward
PELOBiotech launches a revolutionary, truly chemically defined medium
November 2021

© PELObiotech GmbH
Talk from scientist to scientist: Dr. Lothar Steeb, CEO&CSO, and Dr. Peter Frost, CEO PELOBiotech (f.l.t.r.)
“An excellent clinical project starts with excellent manufacturing of cells and media.”
Dr. Peter Frost
CEO PELOBiotech GmbH
Induced pluripotent stem cells, or iPS cells for short, are created in the laboratory but resemble the stem cells of an embryo. Biologists use them to study diseases, doctors in the development of new therapies with the help of patient-specific drug testing. In a few years, more researchers will use stem cells and displace traditional cell culture, experts predict. The global stem cell therapy market is projected to reach USD 401 million by 2026 from USD 187 million in 2021. Market growth is driven mainly by factors such as rising stem cell research activities and increasing approvals of GMP-certified facilities to manufacture stem cells. The increasing demand for iPSCs as an alternative to ESCs and the growing demand for cell & gene therapies are also expected to support the growth of this market in the coming years.
iPS cells can form any cell in the human body. When Japanese Shinya Yamanaka presented his discovery to experts in 2006, it was a revolution; until then, cells had been considered fixed to their development. In 2012, just six years after his discovery, Yamanaka was awarded the Nobel Prize in Medicine. Since then, more and more researchers and doctors around the world are realizing the possibilities offered by research with stem cells. iPS cells are interesting for medical research because they can be used to produce patient-specific cells. Stem cell transplantation has already been used to treat sickle cell anemia and alleviate symptoms of Parkinson’s disease. The demand for new therapies for the treatment of autoimmune, neurological, lung and cardiovascular diseases has resulted in a rise in R&D. Also great, more and more money is being poured into this cell-based research.
“In ten years, we won’t see cell culture of today like this anymore,” says Dr. Peter Frost, CEO PELOBiotech. “I’m hearing more and more that many groups want to replace cell lines and primary cells entirely and switch to stem cell research.” As one of the first members of the German Stem Cell Network (GSCN), PELOBiotech is in regular contact with the leading stem cell researchers worldwide and knows what is needed in the market now and in the future.
Embryonic (ES) and iPS cells are known for their intriguing but challenging nature. Grown without antibiotics and requiring delicate handling, it can take months even years to master the art of iPSC culture. “How to handle these delicate cells are a matter of expertise, knowledge and having access to the right tools,” adds Dr. Lothar Steeb, CSO&CEO PELOBiotech. So, choosing the right product from a wide variety of suppliers can make it a more simply process. For a lot of groups it is also important to switch easily from R&D grade to GMP grade so they might save resources, time and money. That is why PELOBiotech has many high-end Stem Cell culture products, mesenchymal stem cells, media and essential extras in R&D and GMP quality for clinical and industrial research.
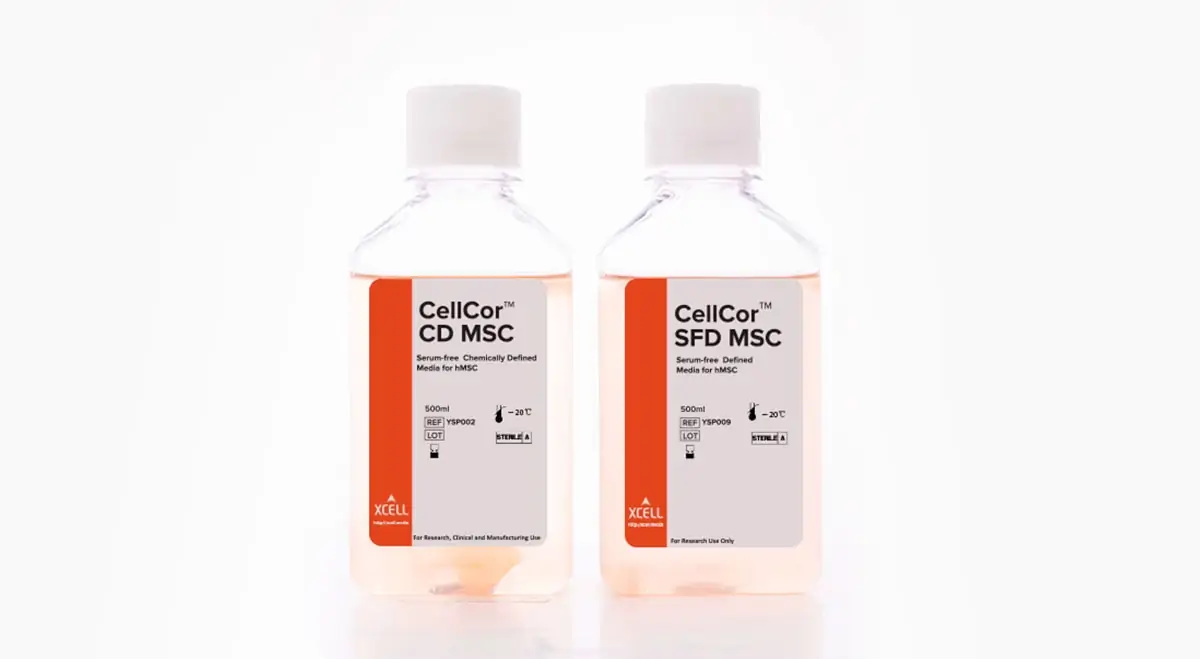
As one of the most interesting products, PELOBiotech is introducing the CellCorTM line into the European market, a one-of-a-kind and truly chemically defined medium. The medium line by Xcell Therapeutics is great for Mesenchymal Stem Cells (MSCs) and available as SFD Serum-free defined MSC Media and Chemically defined MSC Media CellCorTM CD. Important for all clinical and industrial working groups both SC Media are available in R&D and GMP quality. Besides this groundbreaking tool traditional Media for ES/iPS cell culture are crucial to get these cells growing:
Media for Maintenance, Self-Renewal & Differentiation
Special differentiation media are designed to help facilitate the differentiation of iPSC cells into a variety of iPS-derived cells. The Stem Cell expert offer human iPS-derived cells from a variety of donors (e.g. varying donor age and health status) together with specialized media. PELOBiotech has media for ES/iPS cells as well as for iPS-derived cells from famous suppliers like Reprocell and PELOBiotech’s Cellovations brand.
Moving your immuno-research to beyond using Induced Pluripotent Stem Cells (iPS cells)
Pluripotent stem cells are a wonderful tool as through differentiation they can give rise to every other cell type (e.g. cardiomyocytes, hepatocytes as well as neuronal cell types) in the human body, regardless of the original tissue from which they were sourced. PELOBiotech has iPS cells reprogrammed from blood (EPCs), skin (Fibroblast) tissues or urine-derived progenitor cells (UPCs), all with a wide variety of donors. To get more detailed results PELOBiotech can also source human iPS-derived cells from a variety of donors (e.g. varying donor age and health status) with specialized media. The portfolio also includes knock-out animal primary cells and human GFP/RFP tagged cells to meet GMP needs.
© PELObiotech GmbH
Stem, ES/iPS cells and derived products offer great promise for new medical treatments and wide research fields. Get now the big picture of this advanced research field with your Stem Cell experts at your side.
© PELObiotech GmbH
Launched in Europe: The CellCorTM line is chemically defined and consists of 200 known compounds
iPS Reprogramming Tools to become safe, flexible and fast
iPS reprogramming tools can create high quality human iPS cells from multiple target cell types via non-integrating mRNA signaling. The kits aim to deliver safe, flexible, fast and cost-effective reprogramming of both endothelial progenitor cells (EPCs) derived from human peripheral, and cord blood as well as from human adult and neonatal fibroblasts.
As ES/iPS cells have higher demands regarding coating and cryo-preservation, there are specialized tools:
Cryo-Preservation is one of the most important issues in cell culture, specifically in primary and stem cell culture since research with cells depends on viability and shelf-life.
For this purpose, PELOBiotech has collected new and unique formulations of cryo-media especially developed for ES and iPS cells. Classical cryo-media contain additives such as dimethyl sulfoxide (DMSO) which are potential mutagens. Thus, more scientists are now searching for improved cryo-solutions that are DMSO-reduced up to DMSO-free, which in the clinical sector are also available in cGMP Quality. Just to name a few of these media: CryoScarless (Bioverde via Funakoshi, for Stem Cells), ReproCryo (ReproCell, for ES/iPS cells) and CryoNovo P24 (Akron, for MSCs). Beside cells and media, more and more researchers are looking for supporting cell culture tools.
Coating solutions – Extracellular Matrix Solutions (ECM) get cells comfy
Each cell type in the cell culture of both human stem cells and iPS cells needs a tailored setting of physiologically relevant conditions for optimized growth. This growth optimization is provided by ECM proteins through enhancement of cellular functions for cell survival and cell adhesion.
One of the most popular ECM proteins on the market at the moment used in coating solutions is human laminin, specifically the recombinant human laminin-511 E8 fragment, e.g. iMatrix from Reprocell xeno-free and ready to use. Worth a try are definitely iMatrix-511, -411 and -211 (recombinant laminin) And the new gold standard for Stem Cell coating MyMATRIX MSC and MyMATRIX iPSC. Some researchers like to get first an overview which ECM might work best and start with Micro-Matrix™ from MicroStem, a classic Matrix to find out which works best for the set-up. Other variations are also the ready-to-use ECM protein fibronectin (human plasma-derived as well as human recombinant proteins in GMP quality.
CFC/CFU Assays for detailed infos
Assays are a great tool to test a variety of different features and to get more information about one specific detail for your research. In the field of stem cell culture, CFC and CFU assays provide great utility in the identification and enumeration of the different types of functional progenitors in tissues and manipulated cell populations.
Small molecules and growth factors in R&D and GMP-grade products
“We believe in the future of getting more precise research under more and more defined conditions like using serum-, xeno-free or chemically defined tools,” says Dr. Lothar Steeb. To follow this demand there are now a vast array of products available in GMP quality for all your clinical cell culture needs. Small molecules in stem cell culture can be used to research and modulate signaling pathways involved in the generation of iPS cells, and the differentiation of pluripotent stem cells. As the overall interest in serum- or animal-free research is rising, growth factors and cytokines are also available as animal component-free (ACF) and for the clinical demands some even in cGMP quality.
Next to GMP-grade cryo-media, A&AB Serum might also be an alternative for some projects. If scientists are working serum-free there are also virus inactivated human platelet lysate as a substitute for fetal bovine serum. And if you start right from the beginning isolating cells, you might like highly purified enzymes like CIzyme AS for the isolation of ASCs from lipoaspirates (GMP).



