Thermosome’s Lead Program THE001 receives regulatory approval for first-in-human trial
The novel compound will be tested in patients with soft tissue sarcoma.
March 2023
© Thermosome GmbH
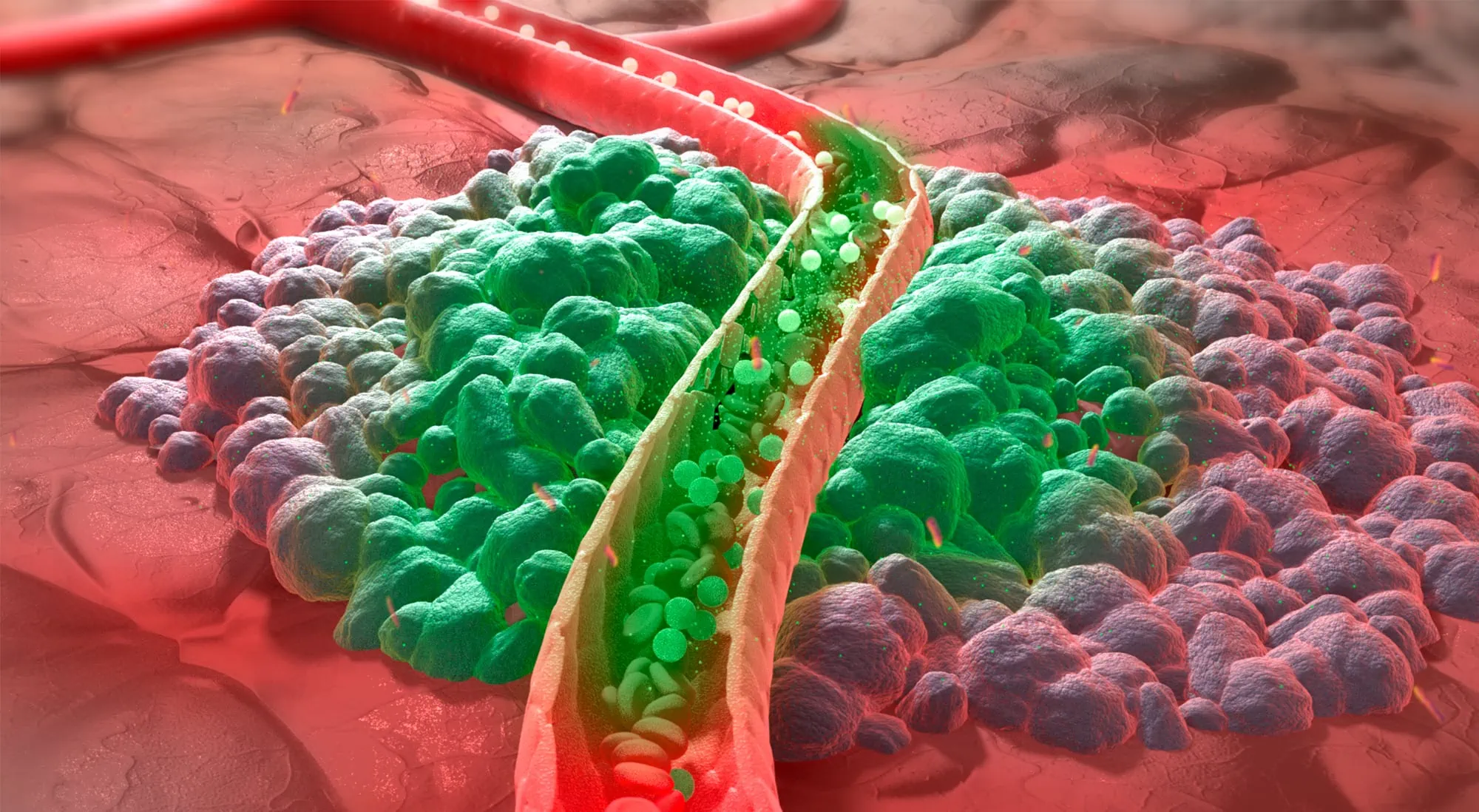
THE001 instantly releases the drug into the blood vessels of the heated tumor, resulting in up to 15-fold increased tumor drug concentration compared to infusion of standard drug.
“This study is an important milestone for our company and the result of a strong team effort. We know from preclinical studies in soft tissue sarcoma that our approach results in significantly improved local efficacy by providing a local boost at the desired site of action.
Dr. Pascal Schweizer, co-founder and CEO/CFO of Thermosome
Thermosome, an IZB-based drug development company specializing in targeted tumor therapies, announced in March that it has received approval from the German Federal Institute for Drugs and Medical Devices (BfArM) and the responsible Ethics Commission to conduct a Phase 1 trial of its lead program THE001 in soft tissue sarcoma in Germany. Patient enrolment is anticipated to begin in the second quarter of 2023.
The primary endpoints of the Phase 1, open-label, interventional dose-escalation study will be the safety and tolerability of THE001 and the determination of the maximum tolerated dose. A secondary objective is to evaluate anti-tumor activity. In the trial, the compound will be administered at three dose levels to patients with locally advanced unresectable or metastatic soft tissue sarcoma, with three to six patients treated per dose level (3+3 design). Patients will be recruited at two clinical sites in Germany specialized on the treatment of soft tissue sarcoma patients: Helios Klinikum Berlin-Buch and LMU Klinikum, Munich. Principal Investigator will be PD Dr. Peter Reichardt, a sarcoma and hyperthermia expert at Helios Klinikum Berlin-Buch.

© Thermosome GmbH
Dr. Pascal Schweizer, Co-founder & CEO/CFO at Thermosome GmbH
THE001 is a thermosensitive liposomal formulation of the chemotherapeutic drug doxorubicin (DPPG2-TSL-DOX). It has a different mode of action than conventional liposomes. Thermosome’s technology enables intravascular drug release initiated by a mild heat trigger using clinically established hyperthermia devices. This results in an up to 15-fold higher local drug concentration and aims to achieve improved clinical treatment efficacy by creating a local boost at the desired site of action. These high local concentrations, which also reach less well perfused areas, aim to overcome drug resistance. This effect cannot be achieved by administration of conventional doxorubicin due to systemic toxicity. Thermosome envisions to further enhance treatment efficacy by an additive immune response induced by regional hyperthermia.
“This study is an important milestone for our company and the result of a strong team effort,” said Dr. Pascal Schweizer, co-founder and CEO/CFO of Thermosome. “We know from preclinical studies in soft tissue sarcoma that our approach results in significantly improved local efficacy by providing a local boost at the desired site of action.”
“I believe that Thermosome´s approach has the potential to change the standard of care for locally advanced soft tissue sarcomas, and I am excited to evaluate THE001 in a clinical setting in an indication with such a high unmet medical need”, added PD Dr. Peter Reichardt, Principal Investigator of the study.
© Thermosome GmbH
3D video explaining the technology of Thermosome
About soft tissue sarcoma (STS)
STS is an atypical tumor with a patient population that includes many young patients. Locally advanced STS (LA-STS) are large invasive tumors that are difficult or impossible to resect. Neoadjuvant therapy is used to shrink these tumors preoperatively and allow tumor surgery with curative intent. Free doxorubicin in combination with ifosfamide or dacarbazine has been the gold standard for neoadjuvant therapy of all chemosensitive LA-STS for several decades. Guidelines also recommend combining DOX-based therapy with regional hyperthermia. Nevertheless, with response rates of less than 30%, there is a significant unmet need for improved treatment options.
Soft tissue sarcomas occur in more than 50 different subtypes, making biologic targeting more difficult than physically controlled targeting with the most active agent. A European orphan drug designation for STS has been granted for THE001.
Thermosome combines targeted tumor therapies with immune stimulation for improved cancer therapy
Thermosome is a clinical-stage drug development company focused on targeted tumor therapy combined with immune stimulation for improved cancer therapy. At its core is a new, proprietary tumor targeting approach, which allows for significantly increased local drug concentrations and improved tumor penetration to achieve improved clinical treatment efficacy.
Thermosome´s approach enables targeted tumor treatment independent of specific molecular targets and covers patient populations across all tumor subtypes. The first clinical indication of its lead drug candidate THE001 is soft tissue sarcoma where the Company aims to improve the current Standard of Care (free doxorubicin).