Neutralizing GDF-15: Development of a new class of anti-cancer immunotherapy
CatalYm combines “good old fashioned efficacious” antibody technology with novel biology
July, 2023
© IZB
IZB Start-up CatalYM Video engl. – new class of anti-cancer immunotherapy
While completing her PhD thesis, Dr. Christine Schuberth-Wagner identified a small RNA based duplex which selectively initiated an innate immune response, and an idea was born. Through further research she realized the duplex could be perfectly suited to provoking an immune response in tumors, the founding idea of Rigontec. Fast forward 14 years and a successful acquisition of Rigontec by MSD in 2017 Christine is now Chief Scientific Officer at CatalYm, an innovative developer of GDF-15 targeting therapies to regulate the immune system in the tumor microenvironment. We spoke to her and CatalYm CEO Dr. Phil L’Huillier, at the company headquarters in the IZB about the small world of biotech innovation and revealing the mysteries of GDF-15.
It’s nice to meet you both and hear a little about how your professional career trajectories led you to CatalYm.
Phil: Yes, it’s an illustration of how small the industry is. You know when we first worked together, I was sitting on the opposite side of the table from Christine as part of a team with MSD sent to assess whether or not her team’s science was worth pursuing and to evaluate the biotech as a potential acquisition target. I was the buyer, Christine was the seller, and the acquisition was a success. We of course didn’t know at that time that we’d end up on the same side of the table in the future. It’s just a nice example of how small the industry is and how important relationships and reputations are.
Christine: I think this is supported by the fact that our two C-level colleagues at CatalYm, Anne Burger und Eugen Leo, are my former colleagues from Rigontec. I covered the pre-clinical efforts as SVP Research at that time. Since we had worked together successfully before, we decided to do it again. So, it is important to identify the people you can perform best with.

© CatalYm
Dr. Christine Schuberth-Wagner

© CatalYm
Dr. Phil L Huillier CEO Catalym
Absolutely. Can you tell us about the research you’re doing now?
Christine: Let’s start with a basic point: tumors need to protect themselves from attack by the human immune system, otherwise they won’t survive. But where can we find a natural role model for providing a powerful defense against immune attacks? The answer is pregnancy. During pregnancy, the fetus expresses paternal antigens which are completely unknown to the maternal immune system. The immune system is trained to deplete foreign antigens from the body, but here the fetus now needs to be protected from the maternal immune system to grow and develop for over nine months in the mother’s body. Within the placenta, multiple molecules function as toolbox of this feto-maternal-tolerance. In cancer we see that tumors use this toolbox of feto-maternal immune tolerance to protect themselves against immune attacks and a number of these toolbox molecules are now very well established targets in the field of immuno-oncology.
In the tolerance toolbox, our scientific founder has identified GDF-15 (“Growth and Differentiation Factor-15”) as an essential cytokine relevant also in the context of cancer. GDF-15 is highly expressed in the placenta by trophoblasts and was already described in 2004 as being one of the key molecules and decisive factors in early days of pregnancy. If newly-pregnant women are unable to produce GDF-15 sufficiently, they will lose their baby. What we now see is that many different tumors express GDF-15. This tells us that tumors mimic this feature of maternal tolerance to protect themselves from immune attack. The CatalYm founder’s lab was the first to establish that connection and to really look at GDF-15 in the context of cancer.
Where are you in clinical development?
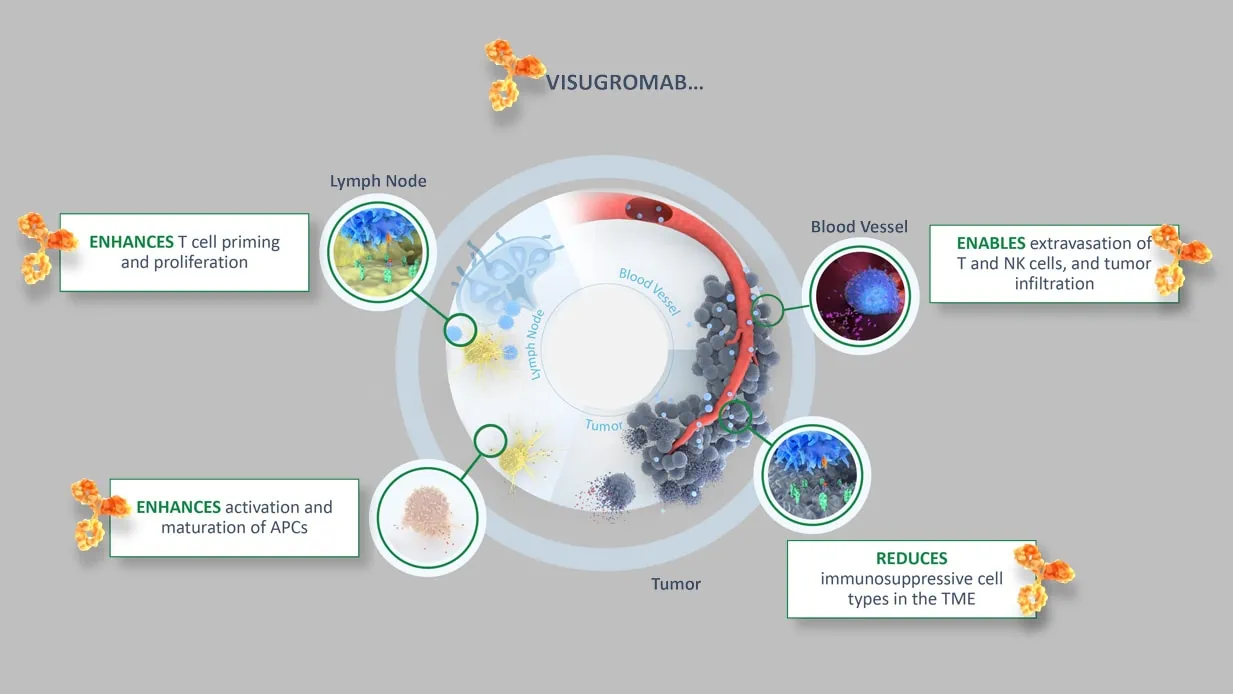
Christine: Our GDF-15 neutralizing monoclonal antibody visugromab is currently in phase 2 testing reading out by end of this year. Visugromab neutralizes GDF-15 in the tumor and blood circulation to reactivate immune cell influx into the tumor and thereby allow patients’ immune systems to respond to or attack tumors and ideally eradicate them. Here we see synergies with already approved therapies like checkpoint inhibitors which are all dependent on sufficient T-cell influx into the tumor. Essentially, we are opening the gates to the immune cells to get into the tumor and kill the tumor cells.
How does this approach differ from others?
Phil: It’s a really novel approach because of the biology associated with the target. The therapeutic modality itself is a fairly standard, good old fashioned monoclonal antibody. This technology has been around for quite a long time and there are many successful drugs on the market that are based on antibody technology. The biology associated with GDF-15 is novel in the way it acts on multiple immune cell types in the tumor microenvironment and in the draining of lymph nodes. That gives it a really essential role as an inhibitor of the anti-tumor immune response.
In non-scientific terms, GDF-15 cloaks the tumor from immune system attack and our antibody uncloaks the tumor so that it can be seen by the immune system and killed.
How does the use of new technologies affect your research? Does this speed up the process or does this just make it deeper and more robust?
Christine: From my perspective, new technologies, in particular RNAseq and bioinformatics make research more precise because they enable us to understand what is going on in an individual patient’s tumor. In addition, they help us understand the driver of disease, and which treatments would be best suited to support the patient. I think this is one of the major advancements in the field and support the trend towards personalized medicine. For this we need to understand the tumor context for each patient, what mutations have occurred, how the tumor-immune microenvironment is configured, which then allows you to better define the treatment that has the highest chance of success for that individual patient.
I imagine the university setting was more academic and IZB is a bit more commercial. Can you talk about that transition and what effect it’s had on your work?
Christine: I think it’s a natural step that you need to take after founding a biotech. What is really great in an academic setting is building the foundation of your research. Building the initial hypothesis and the data that allow you to believe in a concept. But there is a point in time when you need to move on from that and in order to best support a clinical candidate you need non-clinical data which are not necessarily basic science. To do that in a fast-paced and customized way, it’s better to do it in a lab space and with a dedicated research team.
Phil: I agree. It’s very typical for a biotech company to form and start out perhaps in an academic laboratory or start out having licensed IP at the university and then set up on the university site. As the company grows, it needs to bring in different skill sets. We’re now a large clinical-stage biotech company and enjoy the incubator facility at IZB. That works well for us because it’s important that we position the company in the right way to interact with potential partners. We want to build partnerships with other companies, across the globe as we move forward. Also, we have brought on investment from venture capital which requires a professional level of operation.
Tell us about your lab space here.
Christine: We currently have 12 people in the research team (31 people in the company) working in a total of five labs. We started experimentation within two-and-a-half months of moving in. This is indicative of the quality of the lab space and how perfectly suited it is to hit the ground running. In addition, you get into an environment with other biotechs, which creates a unique spirit.
Phil: The local environment of IZB, the ability to talk to or collaborate with others here or close by is really important. As CEO, it’s useful and interesting to be able to talk to my peers with projects in similar stages of development or with similar therapeutic focus. That’s very, very helpful. We’ve got very good facilities. When we bring in board members or external visitors to the site, they all know where it is. Everywhere I go when I say, “We’re a Munich company”, they say “Are you at IZB?”. So, people know the site and lots of investors but also pharma people have been here, and they immediately get a picture of where we’re located.
You’ve had one significant fundraising since you’ve been in the space, right? Can you talk about what the cooperation with investors is like and what might be useful for other biotechs to know about navigating that process?
Phil: We were lucky to have had a good group of supportive investors on board already when we began looking for more. When we started looking to do the C round of financing, the existing investors all agreed to participate in the round significantly. New investors want to know how existing investors are going to support the company. That was the first hurdle and the first vote of confidence in the technology, the management team and in our execution of the program. Then we started conversations with both European and US investors and in the end added two new top tier investors.
It is tough to raise money for early-stage companies or even companies like ours which is relatively advanced. So, my advice is to talk to many investors. But also, investors talk to each other. Certainly, this is a piece of advice for companies in the Munich area where there are a lot of investors now. In my two years here, I have seen the number of European investors opening up offices in Munich has increased. And it’s important to keep in mind there can be a lot of reasons why sometimes they don’t move forward with you. You have to be persistent and recognize that it might not be because of what you’re doing, but for other reasons that they decide at that time not to invest in your company. But that doesn’t mean that in the future they won’t.
What do you need to continue moving forward at CatalYm? What are some upcoming milestones that you’re looking at and what’s your path to achieve those?
Phil: We’re building towards a Phase 2 data package from the clinical studies that are being conducted now. That data package is made-up of a good understanding mechanistically of what GDF-15 does and how it works. It also includes clinical data looking at efficacy, safety and durability of responses and, where we can, the characterization of why some patients respond and some don’t. The next milestone is pulling together all of the discovery data and the clinical data to build that data package. From there, we are planning to move into Phase 2b randomized studies in mid 2024.
I’ve heard a number of people talk about how important data is in this difficult period of fundraising. It sounds like what you’re doing now is right on target. Are there other things you’re doing that you might not have done when the money was flowing more easily?
Phil: You’re absolutely right. Data is key, generating good data, the right data, robust data in a commercial setting is really critical. Articulating the narrative around that data and positioning it alongside competitors and others is also important. Building a strong narrative to communicate your uniqueness, your place in the space and your path forward is important. Establishing relationships with investors and potential pharma partners and getting their feedback is important. You might have closed the funding round quicker and may have had a really nice valuation of that funding round before the slowdown, but it often still took a lot of conversations and you still had to develop a strong story before you went out.
Let’s talk about partnerships. I imagine informal feedback from potential partners can be quite instrumental. How does that work?
Phil: In my view, there is a symbiotic relationship between pharma and biotech. Pharmas have set up systems and processes and teams to know about all that’s going on across the biotech industry. They have people that are interacting with companies like ours, looking to meet companies in the area and learn about our innovation. Their teams are set up to give you feedback about what you’re doing. Sometimes it comes down to the individuals you’re talking to and the relationships you have with them. Face-to-face meetings are important. We’ll go to the JP Morgan conference in San Francisco for example in January so that we can sit down face-to-face with people. That helps build relationships and of course to get better feedback.
Can you tell me what percentage of your time you spend talking to investors?
Phil: Christine would say too much!
Christine: Well, we need to generate data, but one of the important points in our business is to build up active relationships that allow you to call or e-mail somebody and they answer very quickly. It is a big advantage to interactively work with industry insiders with different backgrounds and experiences but building and sustaining the relationships needs a lot of time.
Phil: Yes. For me, it’s probably somewhere like 30-40% of my time. I’m talking about managing relationships with my board, my existing investors, but also pharma and new investor relationships. When we’re in fundraising mode, it also actively involves Christine, our CMO, Eugen Leo and our CFO Anne Burger as well. When we’re all involved it’s probably 60% of our time spent fundraising. I work closely with BD to do a lot of Pharma interactions also. Christine has the academic and discovery interactions and running the team.
I have noticed that when startups begin their work, they think they can do everything on their own and this is just not working. What lessons can you share with startups?
Phil: I think one lesson that I learned a while ago and I have to remind myself still these days is the relationship with your board and your investors is really important. And it’s easy for a management team to say they don’t want the board with the investors too close in what you’re doing. But in fact, you have to have strong relationships and it’s an important part of my job to stay close to my investors and board members and manage the interaction well.
Christine: It’s also important to not miss out on opportunities to interact with academics. At CatalYm we are building a strong scientific and academic collaboration network because we are talking about a new target. How do you spread news and increase the excitement on a novel target? By getting academic groups excited around it, starting research, publishing papers, giving talks, submitting abstracts. By doing that they ignite the fire within the industry and increase interest in and value of your novel approach.
- Am Klopferspitz 19
- 82152 Planegg/Martinsried
- moc.mylatac@ofni
- www.catalym.com