Holistic aproach for drug development
LEUKOCARE makes use of Alchemilla´s innovative approach in the area of Regulatory Affairs and applies it successfully in its projects
June 2019
© Alchemilla
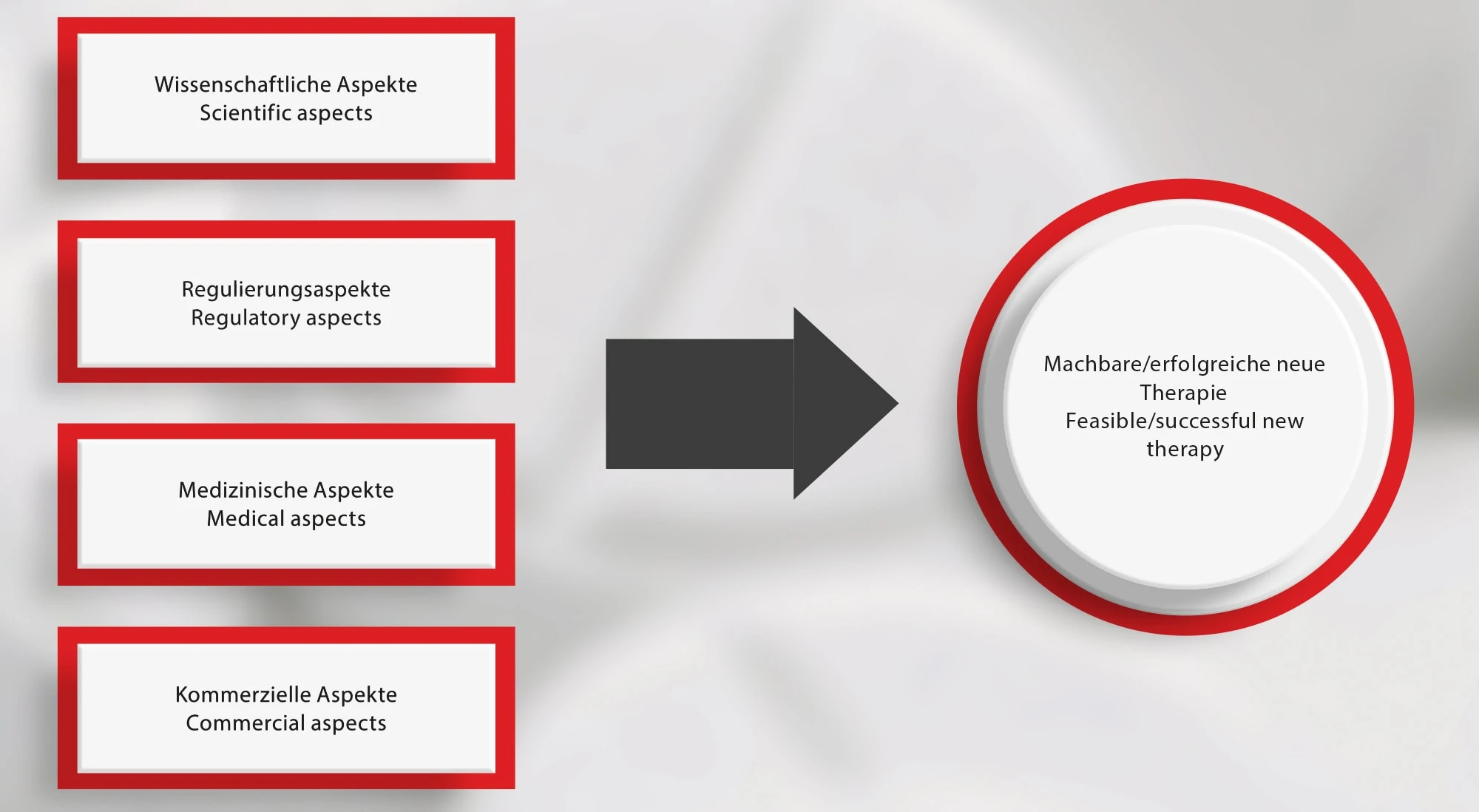
The rising cost of drug development and increasing pressure on drug pricing combined with stretched development timelines continuously challenge current pharma business models. Only about one in ten experimental therapies to start clinical trials makes it to an eventual approval. Roughly 1/4 launches involve drugs that are truly clinically differentiated from competing products and are meeting unmet medical needs. Regulatory issues have also become a major challenge especially for development of new and advanced therapies. Finally, a new and even riskier stage is begging, i.e. drug commercialization. Comprehensive and holistic, scientific, regulatory, medical, and commercial criteria should be diligently considered in very early development phases, which is especially relevant for the smaller biotech and startups.
Alchemilla with its relevant expertise is creating and developing a holistic approach for successful discovery and development of new therapies. LEUKOCARE got to know firsthand how a successful collaboration with Alchemilla looks like: because of Alchemilla´s holistic consulting activities in the area of regulatory affairs in the early phase of development projects, LEUKOCARE was able to overcome regulatory hurdles. In the end, not only LEUKOCARE but also its partners benefited from Alchemilla´s holistic approach.



