Teva and MODAG collaborate on licensing a drug candidate for neurodegenerative diseases
Drug candidate targets disease changes in multiple system atrophy and other neurological disorders like Parkinson
November 2021

© MODAG GmbH
Dr. Torsten Matthias, Chief Executive Officer; Prof. Dr. Armin Giese, Chief Scientific Officer and Prof. Dr. Johannes Levin, Chief Medical Officer (from left to right).
“With the launch of the small molecule drug, we are opening a new chapter in the fight against neurodegenerative diseases and have the opportunity to dramatically improve the lives of millions of patients.”
Torsten Mathias
CEO of MODAG GmbHH
MODAG GmbH, based in the Innovation and Start-up Center Biotechnology (IZB), and Teva Pharmaceutical Industries Ltd. in Tel Aviv announced in October 2021 a strategic collaboration for the exclusive worldwide licensing and development of MODAG’s lead compound anle138b and a related compound, sery433.
The drug candidate anle138b targets pathological alpha-synuclein oligomers and is being evaluated in patients with neurodegenerative diseases for potential disease modification. Under the terms of the agreement and pending regulatory clearance, Teva will receive an exclusive global license to develop, manufacture and commercialize anle138b and sery433. The companies will jointly develop the compounds for the multiple system atrophy (MSA) and Parkinson’s disease (PD) indications based on early-stage clinical studies, and consider exploring additional indications based on clinical outcomes.
Anle138b has the potential to be used in other neurodegenerative diseases, such as Alzheimer’s disease
A Phase 1 (NCT04208152) study examining anle138b in healthy volunteers completed in July 2020 demonstrated favorable benefit-risk profile at all dose levels while achieving higher plasma levels than those required for full therapeutic efficacy in animal models. Anle138b was initially developed in patients with MSA and PD and has the potential to be applied to other neurodegenerative disorders, such as Alzheimer’s disease. A Phase 1b (NCT04685265) clinical trial evaluating the safety of the compound, as well as efficacy measures in patients living with PD, is currently being conducted.
The partnership between MODAG and Teva has the potential to bring a drug for Parkinson’s disease to market
“With Teva’s strong foundation in neuroscience and our in-house expertise in neurology and psychiatry, this licensing and collaboration agreement adds a promising new compound to our early-stage pipeline as a possible orphan disease treatment for the growing patient population living with multiple system atrophy, as well as a potential option for patients living with Parkinson’s disease,” said Hafrun Fridriksdottir, Executive Vice President, Global R&D. “We at Teva are excited about collaborating with the MODAG team and look forward to future developments as we continue to follow the science and explore additional indications for both partnered compounds,” so Fridriksdottir.
Dr. Torsten Matthias, CEO of MODAG added, “We are pleased to partner and work alongside Teva, an organization that has longstanding, extensive expertise in the development of therapeutics. In addition to the previous support, we have received from the Michael J. Fox-Foundation and the Cure Parkinson’s Trust, this partnership further underscores the heightened potential of our lead candidates to do what no drug is currently capable of: blocking the progression of synucleinopathies. Building upon our notable preliminary results, we look forward to the continued development of anle138b alongside Teva to help patients living with currently untreatable neurodegenerative diseases, including MSA, PD and Alzheimer’s disease. With the introduction of small-molecule medication, we open a new chapter in the fight against neurodegenerative diseases and have the chance to improve the lives of millions of patients drastically.”
© Adobe Stock
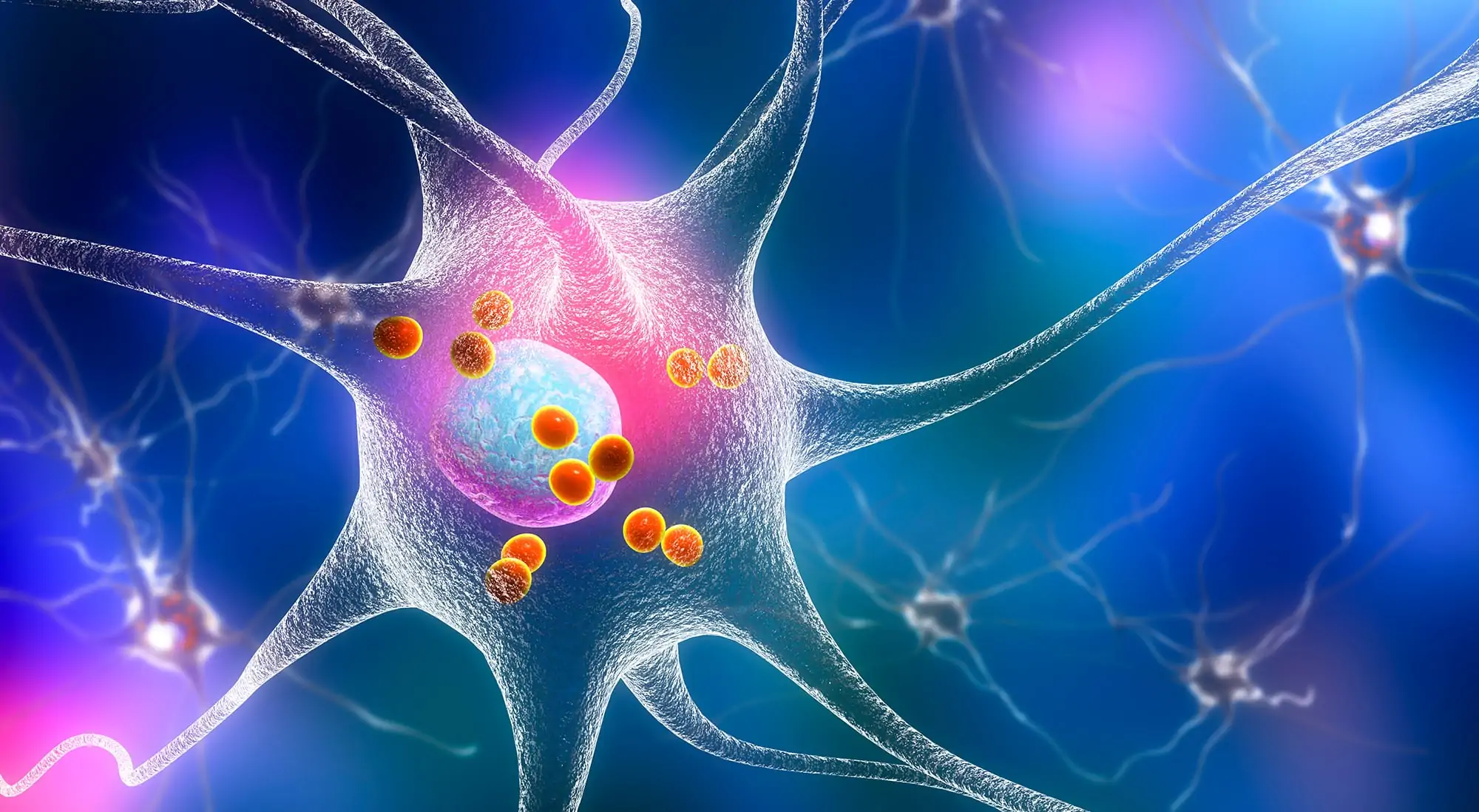
Parkinson’s disease. 3D illustration showing neurons containing Lewy bodies small red spheres which are deposits of proteins accumulated in brain cells that cause their progressive degeneration.
Multiple system atrophy is classified as a rare disease
Multiple system atrophy (MSA) is a rare neurodegenerative disorder classified clinically as “atypical parkinsonism” and belongs to the group of synucleinopathies. MSA is characterized histopathologically by abnormal deposits of the α-synuclein protein, mainly in oligodendroglial cells (glial cytoplasmic inclusions) and also in certain nerve cells. Typically, there is a dysfunction of the autonomic nervous system, i.e., disturbances of bladder function, erectile function, intestinal mobility, or the regulation of blood pressure in combination with a movement disorder. The movement disorder often presents with either Parkinson-like symptoms or a disturbance of cerebellar function, such as ataxia, gait and speech problems. In the US, EU, and Japan, MSA affects about 40,000 people (prevalence of 4 per 100,000), with about 6,000 new cases diagnosed each year (incidence of 0.6 per 100,000). Post-diagnosis life expectancy is about 7-10 years. Due to the relatively small number of affected patients and lack of effective therapy, MSA qualifies for orphan status, allowing a shorter development path. This unmet medical need for MSA disease modification is the driver to develop new therapies that could potentially impact the lives of patients.
Teva Pharmaceutical Industries is a leading global supplier of generic and specialty pharmaceuticals
Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA) has been developing and producing medicines to improve people’s lives for more than a century. We are a global leader in generic and specialty medicines with a portfolio consisting of over 3,500 products in nearly every therapeutic area. Around 200 million people around the world take a Teva medicine every day, and are served by one of the largest and most complex supply chains in the pharmaceutical industry. Along with our established presence in generics, we have significant innovative research and operations supporting our growing portfolio of specialty and biopharmaceutical products. Learn more at www.tevapharm.com.
MODAG GmbH focuses on the research and development of therapeutics and diagnostics for neurodegenerative diseases.
The innovative approach of MODAG offers a unique combination of early diagnosis and targeted disease-modifying therapies for severe neurological disorders. MODAG’s collaborations with the top-tier US and European research institutions, combined with its founders and management team’s interdisciplinary research and development expertise, provide ideal conditions for accelerated implementation of clinical applications. Built upon an extensive portfolio of patented active compounds, MODAG is developing a new oligomer modulator for MSA, PD and other synucleinopathies such as Alzheimer’s disease, intending to deliver novel, first-in-class drugs with the potential to halt disease progression.
MODAG is supported by the following institutions:
Projects funded by the Michael J. Fox Foundation
1. Oligomer Toxicity Inhibition in Synucleinopathies (OTIS):
Evaluation of the oligomer modulator anle138b in a seeding-based primate model of PD (started April 2014)
https://www.michaeljfox.org/grant/evaluation-oligomer-modulator-anle138b-seeding-based-model-parkinsons-disease
MultISyn EU Consortium Multimodal Imaging of Rare Synucleinopathies
Novel PET-tracers to link protein aggregation pathology to functional read-outs in animal models and in the corresponding human patient populations
http://www.multisyn.eu/team.php
The Cure Parkinson’s Trust
https://www.cureparkinsons.org.uk/
ARTEMIS Research Association
http://www.erare.eu/financed-projects/artemis



