LINDIS Biotech develops immunotherapy against bladder cancer
Interview with Dr. Horst Lindhofer, Managing Director LINDIS Biotech GmbH
April 2021
© LINDIS Biotech GmbH
Interview with Dr. Horst Lindhofer, Managing Director LINDIS Biotech GmbH
The biotech company at the Innovation and Start-up Center Biotechnology (IZB) near Munich, Germany, reports the successful start of its Phase I dose escalation clinical trial with the trifunctional antibody CATUMAXOMAB for the treatment of non-muscle invasive bladder cancer (NMIBC). CATUMAXOMAB would be the first specific immunotherapy for non-muscle invasive bladder cancer, an indication with a very high unmet medical need. Treatment of the first dose cohort has already been successfully completed. The study is investigating safety as well as first efficacy signals in up to 30 patients. The results of an interim evaluation are expected as early as fall 2021. Susanne Simon from IZB Biotech News interviewed Dr. Horst Lindhofer, CEO of LINDIS Biotech GmbH, about the development of the new antibody.
© Adobe Stock
Cancer cell
IZB Biotech News: What is the goal of your new immunotherapy?
Dr. Horst Lindhofer: The goal of developing this product candidate CATUMAXOMAB in the indication NMIBC is to reduce the rate of radical bladder removal (cystectomy), as well as to reduce recurrence and progression rates.
IZB Biotech News: What is the advantage of the new antibody?
Dr. Horst Lindhofer: In contrast to the current standard therapy with BCG (Bacillus Calmette-Guérin), CATUMAXOMAB can generate a targeted immune response against the tumor, as the bispecific antibody binds directly to the tumor cell with one of its binding arms. The results from initial investigations have been very motivating and have shown that we could make a real difference in treatment for patients in this indication.
IZB Biotech News: Has this antibody already proven its effectiveness?
Dr. Horst Lindhofer: CATUMAXOMAB is a trifunctional antibody that binds directly to the tumor cell with one of its binding arms and activates two essential components of the immune system with the other binding sites: T cells and macrophages (phagocytes). The antibody recognizes and binds all EpCAM-positive tumor cells, including critical cancer stem cells which express also EpCAM in case of carcinomas. Coming back to your question: Yes, CATUMAXOMAB was already approved for the indication malignant ascites in Europe in 2009, proving its safety and antitumor efficacy in the clinic.
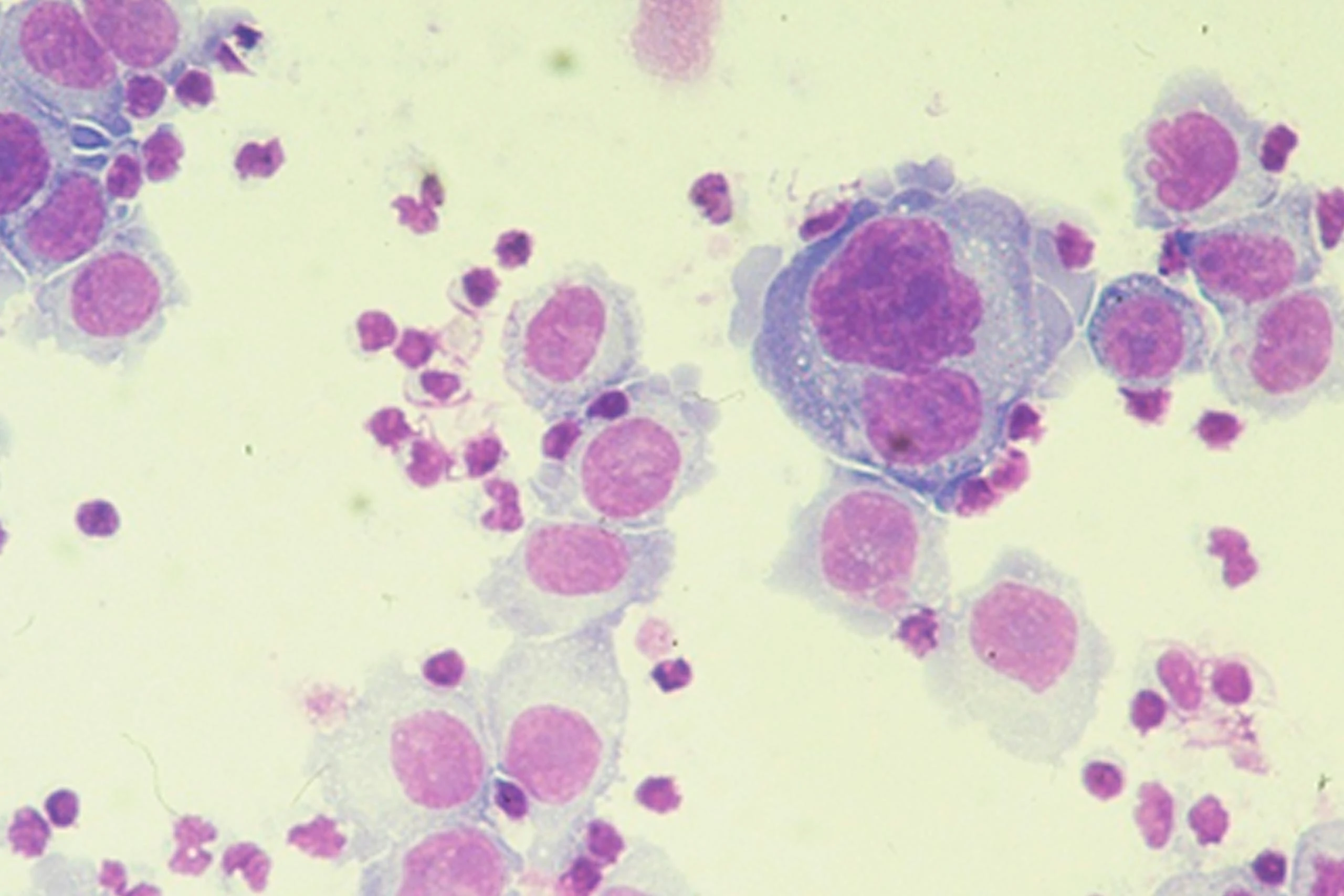
© Riesenberg et al., J.Histochem Cytochem 49:911, 2001
Destruction of urothelial tumor cells using CATUMAXUMAB within 24 hours
without CATUMAXUMAB (24h)
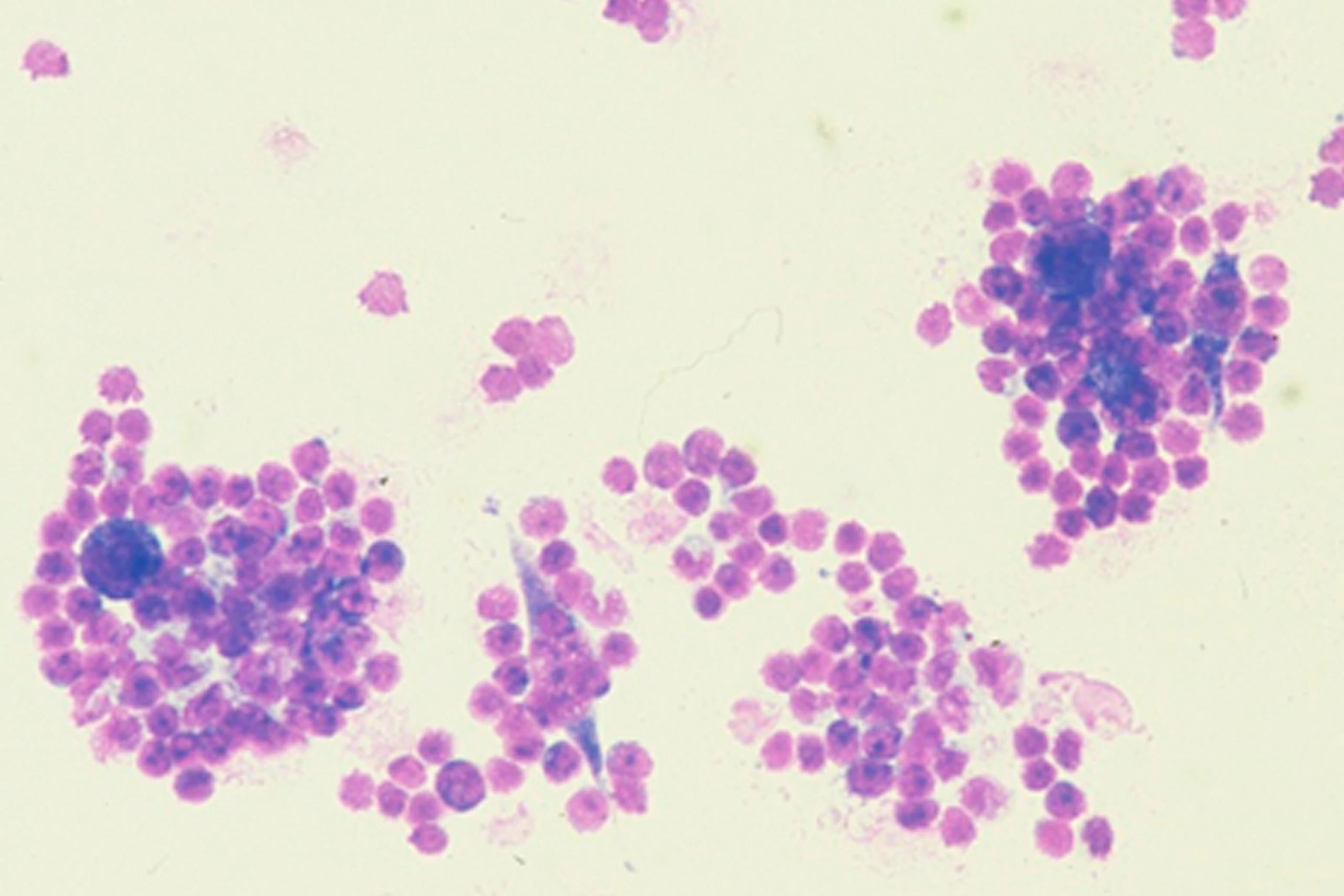
© Riesenberg et al., J.Histochem Cytochem 49:911, 2001
with CATUMAXUMAB (24h)
IZB Biotech News: What is the current standard therapy?
Dr. Horst Lindhofer: NMIBC is a very burdensome cancer for both patients and the healthcare system, as the tumors tend to be multifocal, recur chronically over years, and are usually resistant to chemotherapies. The current standard therapy is a direct injection of BCG (Bacille Calmette Guerin) into the urinary bladder – after surgical tumor reduction, given repeatedly over a period of up to 3 years. If this therapy fails, patients with high-risk NMIBC tumors are often left with only cystectomy to prevent tumor progression, which has a tremendous impact on their quality of life. BCG therapy itself causes painful, non-specific bladder inflammation, which is associated with severe side effects and a high discontinuation rate. There is reasonable hope that with the clinical development of the antibody CATUMAXOMAB, a more effective and tolerable alternative to BCG therapy may be available in the future for the treatment of NMIBC. This would be a major advance in an area where there has been virtually no therapeutic innovation for 20 years.
IZB Biotech News: When did you found LINDIS Biotech GmbH and
what has been reached?
Dr. Horst Lindhofer: I founded LINDIS Biotech GmbH in 2010. The basis of the biopharmaceutical company is the proprietary multispecific antibody platform. LINDIS has an advanced development pipeline with three clinical product candidates in immuno-oncology. We are the only company with a technology that combines highly effective tumor cell destruction with patient-specific vaccination based on trifunctional bispecific antibodies, and therefore occupies a prominent position in the field of cancer immunotherapeutics. As the first product to emerge from this platform, CATUMAXOMAB was approved under the name Removab® for the indication malignant ascites in Europe back in 2009, demonstrating its safety and antitumor efficacy in the clinic – a breakthrough in the development of bispecific antibodies.

© LINDIS Biotech GmbH
Dr. Horst Lindhofer, Managing Director LINDIS Biotech
- Am Klopferspitz 19
- 82152 Planegg/Martinsried
- ed.hcetoibsidnil@ofni
- www.lindisbiotech.com